Abstract
Background: Most pts with MM will be exposed to alkylators throughout their disease course as part of a multidrug regimen or as high-dose melphalan (HDM) conditioning prior to undergoing an autologous stem cell transplant (SCT). MM invariably relapses and can become refractory to prior treatments. Outcomes are poor for pts with RRMM, highlighting the need for effective agents with novel mechanisms of action (Kumar et al. Leukemia. 2017;31:2443).
Melphalan flufenamide (melflufen) is a first-in-class peptide-drug conjugate (PDC) that targets aminopeptidases and thereby rapidly releases alkylating agents inside tumor cells. Previously reported analyses from the phase 2 HORIZON study of melflufen + dex in pts with RRMM show that melflufen is active in pts who have prior exposure to alkylator therapy, and safety signals were not impacted by prior alkylator exposure (Rodriguez-Otero, et al. ASCO . 2021; Poster 8048). Topline results from the phase 3 randomized OCEAN study demonstrated the primary endpoint was met, with superior progression-free survival (PFS) for melflufen + dex vs pom + dex in pts with RRMM (hazard ratio [HR] 0.79 [95% CI, 0.64-0.98]) (Oncopeptides. Press release. July 8, 2021). In this analysis, the clinical activity of melflufen is examined in a subset of pts from OCEAN exposed to prior alkylator therapy.
Methods:
Eligible pts with RRMM had received 2-4 prior lines of therapy (LoTs), including lenalidomide (len) and a proteasome inhibitor, and were refractory to len (within 18 mo of randomization) and to the last LoT. Pts were stratified by age, number of prior LoTs, and International Staging System score, and were randomized 1:1 to receive 28-d cycles of melflufen 40 mg intravenously (d1) or pom 4 mg orally (PO; daily, d1 to 21); all pts received dex 40 mg (20 mg for pts ≥75 y) PO on d1, 8, 15, and 22. Pts were treated until disease progression or unacceptable toxicity. The primary endpoint was PFS. Key secondary endpoints were overall survival (OS), overall response rate (ORR), and safety. Refractoriness was defined as disease that failed to achieve a minimal response or progressed while on primary or salvage therapy or within 60 d of last dose. Efficacy was analyzed by alkylator exposure or refractory status with/without SCT in prior LoTs.
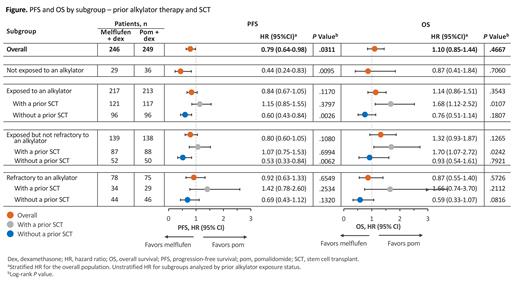
Results: At the data cutoff of Feb 3, 2021, 495 pts were randomized to receive melflufen (n=246) or pom (n=249) in the intention-to-treat population, with 125 pts (51%) and 120 pts (48%) having received a prior SCT. In the melflufen and pom groups, 29 pts (12%) and 36 pts (14%) were not exposed to a prior alkylator, whereas 217 pts (88%) and 213 pts (86%) were exposed to prior alkylators; 139 (57%) and 138 (55%) were exposed to a prior alkylator but not refractory and 78 (32%) and 75 (30%) were refractory to a prior alkylator, respectively. The majority of pts exposed to prior alkylators received cyclophosphamide (melflufen, 59%; pom, 58%), standard dose melphalan (melflufen, 25%; pom, 26%), and/or HDM (melflufen, 46%; pom, 44%).
Melflufen was associated with increased PFS compared with pom in pts not exposed to prior alkylators (HR, 0.44 [95% CI, 0.24-0.83]) and in pts without a prior SCT who were exposed to an alkylator (HR, 0.60 [95% CI, 0.43-0.84]) or exposed but not refractory to an alkylator (HR, 0.53 [95% CI, 0.33-0.84]), with a trend for increased PFS in pts refractory to an alkylator without a prior SCT (HR, 0.69 [95% CI, 0.43-1.12]; Figure). Similar trends in OS were seen in in pts without a prior SCT who were exposed to an alkylator (HR, 0.76 [95% CI, 0.51-1.14]) or refractory to an alkylator (HR, 0.59 [95% CI, 0.33-1.07]). In pts with a prior SCT, pom was generally associated with improved PFS and OS, regardless of alkylator exposed or refractory status. ORR was significantly higher in the melflufen group compared with the pom group for pts not exposed to prior alkylators (59% vs 22%; P=.0029) and numerically higher in pts without prior SCT who were exposed (38% vs 28%; P=.1677), exposed but not refractory (44% vs 32%; P=.2061), and refractory (30% vs 24%; P=.5481) to prior alkylators.
Conclusion:
Melflufen + dex was associated with higher PFS, OS, and ORR compared with pom + dex in pts with RRMM without prior SCT, but not in pts with prior SCT. These results support the use of melflufen + dex in pts with RRMM without prior SCT, including pts who are exposed or refractory to prior alkylators, without HDM exposure from prior SCT.
Sonneveld: SkylineDx: Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding. Mateos: AbbVie: Honoraria; Regeneron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sea-Gen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bluebird bio: Honoraria; GSK: Honoraria; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene - Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees. Robak: Amgen: Honoraria; Janssen: Honoraria; Celgene: Honoraria, Research Funding; Medical University of Lodz: Current Employment. Schjesvold: AbbVie: Honoraria; Adaptive Biotechnologies: Consultancy; Schain: Honoraria; Bayer: Consultancy; SkyliteDX: Honoraria; Oncopeptides: Consultancy, Current holder of individual stocks in a privately-held company, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Nordics Nanovector: Current holder of individual stocks in a privately-held company; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria. Hajek: Janssen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharma MAR: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Research Funding; AbbVie: Consultancy, Honoraria. Richardson: Sanofi: Consultancy; AbbVie: Consultancy; Karyopharm: Consultancy, Research Funding; Oncopeptides: Consultancy, Research Funding; AstraZeneca: Consultancy; Protocol Intelligence: Consultancy; GlaxoSmithKline: Consultancy; Secura Bio: Consultancy; Janssen: Consultancy; Takeda: Consultancy, Research Funding; Regeneron: Consultancy; Celgene/BMS: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding. Harmenberg: Oncopeptides AB: Consultancy, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Other: Travel, Accommodations, Expenses . Thuresson: Oncopeptides AB: Consultancy, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Byrne: Oncopeptides AB: Current Employment, Current holder of stock options in a privately-held company; BMS: Current holder of individual stocks in a privately-held company. Dimopoulos: BMS: Honoraria; Janssen: Honoraria; Beigene: Honoraria; Takeda: Honoraria; Amgen: Honoraria.
Yes, this abstract includes a subgroup analysis of a phase 3 investigational study of melflufen in patients with RRMM refractory to lenalidomide